Abstract
Background: Myelodysplastic syndromes (MDS) are a heterogenous group of hematopoietic stem cell malignancies characterized by ineffective blood cell production. Treatment can include frequent blood transfusions, which may lead to iron overload and subsequent complications. Several treatment guidelines recommend iron chelation therapy (ICT) for lower-risk patients with MDS based on transfusion burden, ferritin level, or iron overload imaging studies. However, ICT options are limited for patients with MDS because deferoxamine is administered parenterally and requires a burdensome infusion regimen that may reduce treatment adherence. Deferasirox is administered orally and requires closely monitoring a patient's renal and hepatic function; deferasirox is contraindicated in patients with significant renal impairment (estimated glomerular filtration rate <40 mL/min/1.73m2), a frequent comorbidity in the primarily geriatric population of patients with MDS. Deferiprone (DFP) is an oral ICT approved for transfusional iron overload in thalassemia, sickle cell disease, and other anemias. Although DFP has no contraindication or dosing adjustment based on renal function, it requires close patient monitoring and laboratory testing, since its use is associated with severe neutropenia (absolute neutrophil count <0.5x109/L) in 1-2% of patients. While existing studies have evaluated the effects of DFP in patients with MDS, limited data on its safety are available for this patient population.
Objective: To evaluate the DFP safety profile in patients with MDS from a US safety registry and a compassionate use program.
Methods: The DFP US safety registry was established to meet FDA postmarketing requirements following DFP approval in the US in 2011. The registry assessed all reports of adverse events (AEs) in all patients, including those with MDS, from 2011 to 2021. The compassionate use program included patients in the US and Canada from 1996 to 2015. All AEs were assessed in all patients with MDS who received this chelator. In both the safety registry and the compassionate use program, DFP was administered at doses that ranged from 75 - 99 mg/kg/day. AEs were assessed irrespective of a causal relationship with DFP.
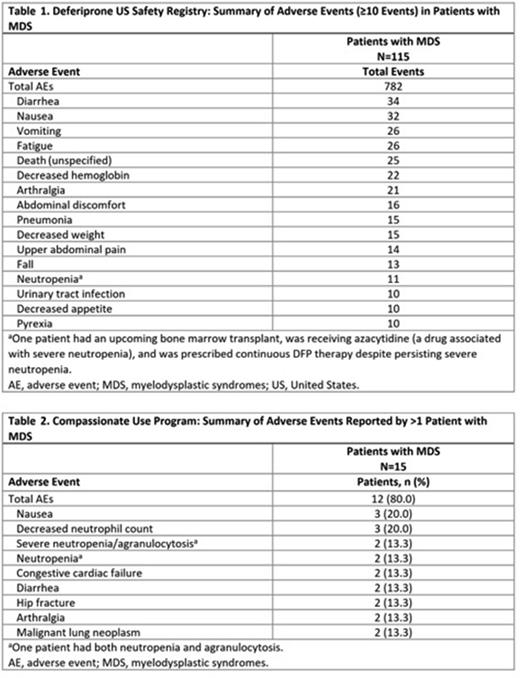
Results: The US safety registry included 115 adults with MDS (mean ± SD age: 77.7 ± 9.0 y; 61.7% males). Mean ± SD DFP exposure was 1.1 ± 1.4 y (range: 0-7.9 y), with 59 (51.3%) patients receiving DFP for > 6 mo and 34 (29.6%) for > 12 mo. Reasons for discontinuation (> 1 patient) included death (n=30), physician direction (n=26), adverse reaction (n=16), hospice care (n=12), nonresponsive patient (n=6), insurance issues (n=4), and switching to another chelator/formulation (n=3). Observed AEs are summarized in Table 1. The most common AEs were diarrhea (n=34), nausea (n=32), vomiting (n=26), and fatigue (n=26). No deaths were attributed to DFP. In 3 patients, neutropenia was reported in temporal association with fatal outcome, but none were attributed to DFP. Four patients (3.5%) reported severe neutropenia/agranulocytosis: 2 events resolved; 1 case outcome was not reported; and 1 case was ongoing at last follow-up.
The compassionate use program included 15 adults with MDS (mean ± SD age: 67.9 ± 10.4 y; 60% males). Mean ± SD DFP exposure was 1.2 ± 2.3 y, with a median (range) of 0.7 y (0-9.1 y). Reported AEs are summarized in Table 2. The most common AEs were nausea and decreased neutrophil count (n=3 each). There were 2 cases of severe neutropenia/agranulocytosis; both resolved.
Conclusions: The findings of the safety registry and a compassionate use program suggest the DFP safety profile in MDS is potentially consistent with that of thalassemia, sickle cell disease, and other anemias. No unexpected AEs and no new safety concerns were found. The frequency of the most serious AE associated with DFP, severe neutropenia/agranulocytosis, was 3.5% within this population; of reported outcomes, all resolved. In the compassionate use program, 13.3% of patients reported agranulocytosis, which is higher than what has been shown in patient populations without MDS (2%); however, it is not clear if this percentage is higher than other ICT agents used in MDS, due to the patients' primary disease, or the relatively small sample size. While acknowledging these limitations and observational nature of the data, DFP may be a viable ICT for patients with lower-risk MDS.
Disclosures
Zeidan:Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding. Fradette:Chiesi Canada Corp: Current Employment. Rozova:Chiesi Canada Corp: Current Employment. Toiber Temin:Chiesi Canada Corp: Current Employment. Tricta:Chiesi Canada Corp: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal